We serve Lithium bis(trifluoromethanesulphonyl)imide CAS:90076-65-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
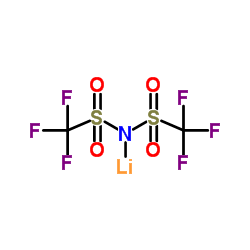
Contact us for information like Lithium bis(trifluoromethanesulphonyl)imide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Lithium bis(trifluoromethanesulphonyl)imide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Lithium bis(trifluoromethanesulphonyl)imide Use and application,Lithium bis(trifluoromethanesulphonyl)imide technical grade,usp/ep/jp grade.
Related News: There are no reliable vaccines available to rid your body of a coronavirus.2-Bromo-3-chloro-5-nitropyridine manufacturer Italy and Vietnam included Taiwan in banning flights from China, a move that they announced after the W.H.O. declared the coronavirus outbreak a global health emergency.2-Deoxy-2,2-difluoro-D-ribofuranose-3,5-dibenzoate supplier According to statistics, in 2017, China’s total production of chemical drugs reached 3.478 million tons, a year-on-year increase of 1.6%. The main business income showed a steady increase, from 328.972 billion yuan in 2012 to 573.475 billion yuan in 2017. The total profit was 48.644 billion yuan, but profit margins remain low (8.48% in 2017).2-Methyl-4'-(methylthio)-2-morpholinopropiophenone vendor As United Airlines said when suspended flights to Beijing, Shanghai and Chengdu, the decision was made “due to the continued drop in demand for travel to China and the US Department of State’s decision to raise its China travel advisory to a Level 4.”The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.

