We serve Methanesulfonic acid 4,4,5,5,5-pentafluoro-pentyl ester CAS:252947-01-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
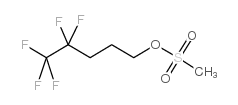
Contact us for information like Methanesulfonic acid 4,4,5,5,5-pentafluoro-pentyl ester chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,methanesulfonic acid 4,4,5,5,5-pentafluoro-pentylester physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Methanesulfonic acid 4,4,5,5,5-pentafluoro-pentyl ester Use and application,methanesulfonic acid 4,4,5,5,5-pentafluoro-pentylester technical grade,usp/ep/jp grade.
Related News: At present, API companies basically use multi-functional and multi-purpose workshops, and the main equipment uses multi-functional reactors to achieve flexible utilization of production capacity.2-Hydroxy-3-methoxy-3,3-diphenylpropanoic acid manufacturer The market for pharmaceutical intermediates is vast and is expected to usher in new growth. Compared with the pesticide intermediate business, the market space for pharmaceutical intermediates is larger and will be the focus of the company’s future development.2-(3-ethoxy-4-methoxyphenyl)-1-(methylsulfonyl)eth-2-ylamine supplier The market for pharmaceutical intermediates is vast and is expected to usher in new growth. Compared with the pesticide intermediate business, the market space for pharmaceutical intermediates is larger and will be the focus of the company’s future development.methyl 3-amino-4,4-dimethoxybut-2-enoate vendor The market for pharmaceutical intermediates is vast and is expected to usher in new growth. Compared with the pesticide intermediate business, the market space for pharmaceutical intermediates is larger and will be the focus of the company’s future development.The CRL for the Cabenuva injection, containing two active ingredients cabotegravir and Janssen’s rilpivirine, follows U.S. market approval in April for its once-a-day pill Dovato, also a two-drug combination.

