We serve Methyl 2-amino-4-((2,5-dichlorophenyl)carbamoyl)benzoate CAS:59673-82-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
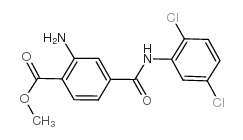
Contact us for information like Methyl 2-amino-4-((2,5-dichlorophenyl)carbamoyl)benzoate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Methyl 2-amino-4-(((2,5-dichlorophenyl)amino)carbonyl)benzoate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Methyl 2-amino-4-((2,5-dichlorophenyl)carbamoyl)benzoate Use and application,Methyl 2-amino-4-((2,5-dichlorophenyl)carbamoyl)benzoate technical grade,usp/ep/jp grade.
Related News: The Company’s immuno-oncology product candidates include natural killer (NK) cell and T-cell cancer immunotherapies, which are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies, and to target tumor-associated antigens with chimeric antigen receptors (CARs).Di(pyrrolidin-1-yl)methanone manufacturer Professor Coleman was involved in some of the first research into MERS in the US.Ethyl acetoacetate supplier Therefore, the R & D capabilities and registration capabilities of API companies have become the core factors of competition.2-fluoro-4-methyl-3-nitropyridine vendor The bulk-buy program, which currently covers 25 types of medicines, allows no more than three successful bidders access to China’s public hospitals, where most Chinese people buy their drugs.These mathematical dosing algorithms were developed in the Damiano Lab at Boston University and refined based on results from home-use clinical trials in adults and children with T1D. Beta Bionics is a Certified B Corporation? whose founders — in addition to Ed Damiano — include other parents of children with type 1 diabetes and people with type 1 diabetes. Beta Bionics is committed to acting in the best interests of the diabetes community and to profoundly disrupting the diabetes medical device industry by bringing the iLet to market as expeditiously and responsibly as possible.

