We serve Methyl 3-methylthiopropionate CAS:13532-18-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
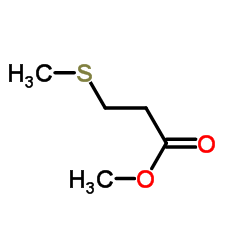
Contact us for information like Methyl 3-methylthiopropionate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Propanoic acid, 3-(methylthio)-, methyl ester physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-(Methylthio)propionic Acid Methyl Ester Use and application,3-(Methylthio)propionic Acid Methyl Ester technical grade,usp/ep/jp grade.
Related News: China has asked the European Union for help in buying urgently needed medical supplies from its member countries, the China’s official Xinhua news agency said on Saturday.2-(4-aminomethylphenyl)benzamide manufacturer In these situations, the API is not a single substance but the culmination of various ingredients.L-alpha-Amino-n-butyric acid supplier In these situations, the API is not a single substance but the culmination of various ingredients.3-Methoxybenzeneboronic Acid vendor These include: high-barrier generic drug substances and commonly used generic drug substances.Biogen last month revived its plans to seek U.S. approval for its aducanumab treatment after announcing in March that it would terminate two large clinical trials for the drug. But some analysts believed FDA approval is highly unlikely.

