We serve Myristoyl Tetrapeptide-12 CAS:959610-24-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
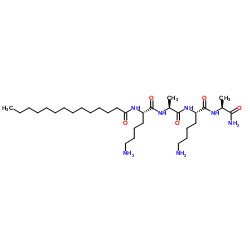
Contact us for information like Myristoyl Tetrapeptide-12 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,N2-(1-Oxotetradecyl)-L-lysyl-L-alanyl-L-lysyl-L-alaninamide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Myristoyl Tetrapeptide-12 Use and application,Myristoyl Tetrapeptide-12 technical grade,usp/ep/jp grade.
Related News: In the process of fine chemical industry transfer, intermediates are a very important category, and they are also the main link for China to undertake transfers.Fmoc-beta-cyclohexyl-D-alanine manufacturer In the process of fine chemical industry transfer, intermediates are a very important category, and they are also the main link for China to undertake transfers.(1S)-1-[3,5-bis(trifluoromethyl)phenyl]ethanol supplier Domestic API companies cooperate with major international companies through commissioned processing, patent transfer, and joint venture production.3-Piperazinobenzisothiazole Hydrochloride vendor Here’s what we can tell you so far:We are pleased that intravenous rigosertib will be made compliantly available to suitable patients with higher-risk MDS through their physicians in designated countries.

