We serve n-octanoyl-dl-homoserine lactone CAS:106983-30-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
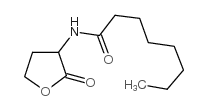
Contact us for information like n-octanoyl-dl-homoserine lactone chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-octanoylamino-dihydro-furan-2-one physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-octanoylamino-dihydro-furan-2-one Use and application,N-octanoylhomoserine lactone technical grade,usp/ep/jp grade.
Related News: The foundational patent, which expires in 2034, is owned by MSK and is licensed exclusively to Fate Therapeutics for all human therapeutic uses.Benzaldehyde, 4-[[4-(dodecyloxy)phenyl]methoxy]-2-hydroxy- manufacturer Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2).2,6-ditert-butyl-4-prop-1-en-2-ylphenol supplier Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2).1-[2-bromo-1-(1-methylprop-2-ynyloxy)ethyl]-3,5-dimethyl-1H-pyrimidine-2,4-dione vendor Immediately thereafter, the iLet begins controlling blood-sugar levels automatically, without requiring the user to count carbohydrates, set insulin delivery rates, or deliver bolus insulin for meals or corrections.It has reserves in the direction of APIs and intermediates for lowering blood lipids, lowering blood sugar and anticoagulation.

