We serve Nesiritide acetate CAS:114471-18-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
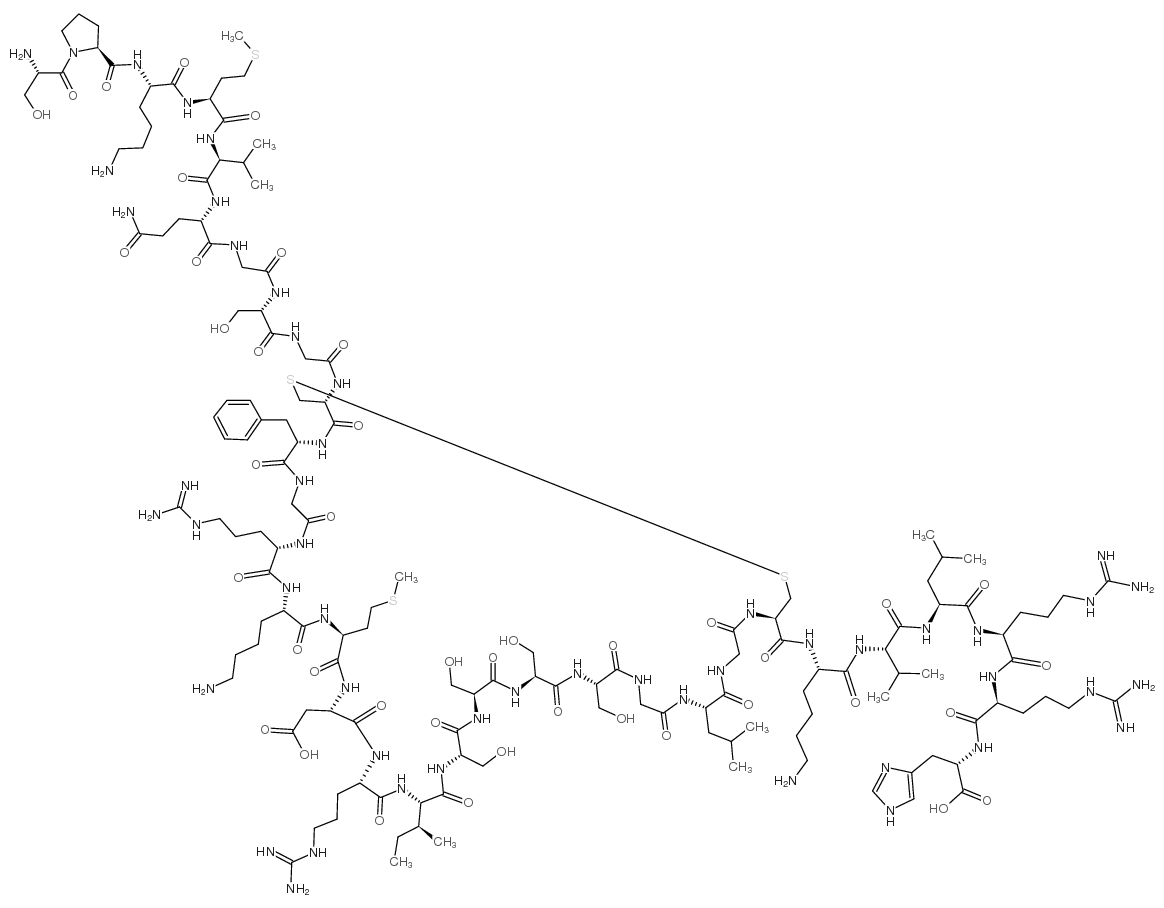
Contact us for information like Nesiritide acetate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,BNP (1-32),HUMAN physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Nesiritide acetate Use and application,BNP (1-32),HUMAN technical grade,usp/ep/jp grade.
Related News: New Zealand citizens and permanent residents and their immediate family members will still be able to enter — but must self-isolate for 14 days after arriving back into the country.Phenyltrimethoxysilane manufacturer The monthly injection to suppress the virus that causes AIDS was aimed as an alternative to daily pills.2-Fluoro-6-nitrobenzoic acid supplier The monthly injection to suppress the virus that causes AIDS was aimed as an alternative to daily pills.2-Fluoro-3-nitropyridine vendor After testing, our quality assurance staff confirms that everything has been performed correctly in accordance with GMP from manufacturing of API to quality testing.INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment.

