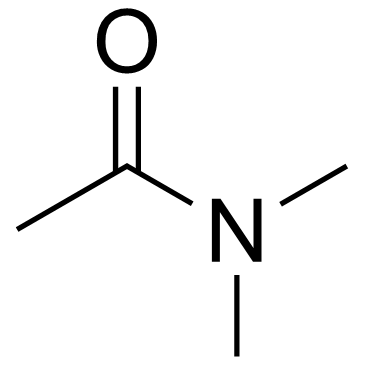
We serve N,N-dimethylacetamide CAS:127-19-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
| Items of Analysis | Standard of Analysis | Test Results |
| Appearance | Colorless and Clear liquid | Colorless and Clear liquid |
| Water Content | ≤300ppm | 240ppm |
| Acidity base on acetic acid | ≤200ppm | 80ppm |
| Basicity base on Dimethylamine | ≤100ppm | 28ppm |
| PH (25℃,20% aqueous solution) | 4.0~8.0 | 6.8 |
| Relative Density (20℃) | 0.938~0.942 g/ml | 0.94 g/ml |
| Assay | ≥99.5% | 99.83% |
| Conclusion | Conforms to Factory Standard | |
Contact us for information like N,N-dimethylacetamide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,N,N-dimethyl-acetamide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,N,N-Dimethylethanamide Use and application,Dimethylamide acetate technical grade,usp/ep/jp grade.
Related News: “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.2-(9H-fluoren-9-ylidene)-1,3-dithiane manufacturer “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.Ammonium hexacyanoferrate hydrate supplier “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.2-bromo-8,8-dimethyl-5,6,7,8-tetrahydronaphthalene vendor From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.

