We serve N,N-Dimethylallylamine CAS:2155-94-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
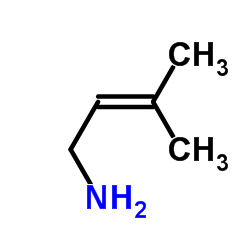
Contact us for information like N,N-Dimethylallylamine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,N-ALLYDIMETHYLAMINE physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,ALLYLDIMETHYLAMINE Use and application,ALLYLDIMETHYLAMINE technical grade,usp/ep/jp grade.
Related News: Under the plan, nonessential hospital staff members who belong to the union would not go to work on Monday.3,4,5-Trimethoxyaniline manufacturer Pharmaceutical intermediates: chemical raw materials or chemical products used in the process of pharmaceutical synthesis, are intermediate products in the process of producing APIs, and can be further processed into APIs.(S)-3-(1-AMINO-ETHYL)-PHENOL supplier Airlines operating in the United States will be required to ask all passengers booked on flights from outside the US if they’ve been to mainland China in last 14 days.4-Fluoro-2-methylbenzonitrile vendor DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation.The on-body wear is similar to that of an insulin pump. Unlike insulin pump therapy, however, the investigational system is designed such that users enter only their body weight for the iLet to initialize therapy.

