We serve O-Desmorpholinopropyl Gefitinib CAS:184475-71-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
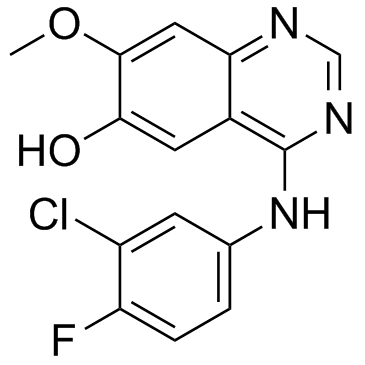
Contact us for information like O-Desmorpholinopropyl Gefitinib chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-ol physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-ol Use and application,4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-ol technical grade,usp/ep/jp grade.
Related News: The term active pharmaceutical ingredient may refer to an active chemical within an FDA-regulated drug, or API might mean the entire drug with its active and inactive ingredients.4'-Methyl-2-cyanobiphenyl manufacturer Russia, which shares a 2,600-mile border with China, reported its first two coronavirus cases on Friday.oxolan-2-ylmethyl 2-methylprop-2-enoate supplier The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.2-FLUORO-5-HYDROXYPYRIDINE vendor The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.”Traditional medicine is a treasure of Chinese civilization embodying the wisdom of the nation and its people,” Xi said at the meeting in Beijing.

