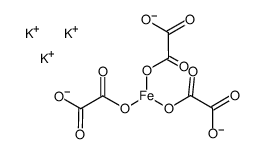
We serve POTASSIUM TRIOXALATOFERRATE(III) CAS:5936-11-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
| Items of Analysis | Standard of Analysis | Test Results |
| Appearance | Yellow green crystal | Conform |
| Purity | ≥99.0% | 99.4% |
| PH | 4.2~5.5 | 4.92 |
| Conclusion | Conforms to Factory Standard | |
Contact us for information like POTASSIUM TRIOXALATOFERRATE(III) chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,POTASSIUM TRIOXALATOFERRATE(III) physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,POTASSIUM TRIOXALATOFERRATE(III) Use and application,POTASSIUM TRIOXALATOFERRATE(III) technical grade,usp/ep/jp grade.
Related News: Common signs of infection include respiratory symptoms, fever, cough, shortness of breath and breathing difficulties. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure and even death. 2-Methyl-6-oxo-4-phenyl-1,4,5,6-tetrahydro-pyrimidine-5-carboxylic acid isopropyl ester manufacturer Mankind Pharmaceuticals on Monday said it has inked a licensing pact with Glenmark Pharmaceuticals to co-market diabetes drug Remogliflozin Etabonate in the country. (S)-3-methyl-2-butylboronic acid supplier Inceptua has global operations with local offices across Europe, USA, and Asia(S)-N-[[3-(3-fluoro-4-(1,2,4-triazolyl)phenyl)-2-oxo-5-oxazolidinyl]methyl]-4-(2-naphthyl)-4-oxobutanamide vendor ViiV, in which Pfizer and Shionogi have small stakes, said it received a so-called complete response letter (CRL) from the FDA in which the regulator questioned the treatment’s chemistry, manufacturing and controls process, but not its safety.The drug review center will establish a registration platform and database for APIs, pharmaceutical excipients and pharmaceutical packaging materials, which means that the domestic drug substance DMF system is expected to be gradually implemented.

