We serve Propylphosphonic Acid Anhydride CAS:68957-94-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
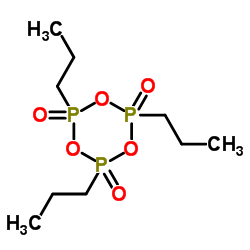
Contact us for information like Propylphosphonic Acid Anhydride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,4,6-Tripropyl-1,3,5,2,4,6-trioxatriphosphinane 2,4,6-trioxide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,4,6-Tripropyl-1,3,5,2,4,6-trioxatriphosphinane 2,4,6-trioxide Use and application,Propylphosphonic Acid Anhydride technical grade,usp/ep/jp grade.
Related News: Further research on Oligomannate’s pharmacological mechanism and long-term safety and effectiveness is required, according to the NMPA statement.1-chloro-2-methyl-3-methylsulfanylbenzene manufacturer However, pharmaceutical intermediates are subdivided into primary intermediates and advanced intermediates. Because primary intermediate suppliers can only provide simple intermediate production, they are at the front end of the industrial chain. The pressure of competition and price is the greatest. The price fluctuations of basic chemical raw materials have a greater impact on them.Diiodomethane supplier A key publication in a preclinical model demonstrated rigosertib’s ability to block cellular signaling by targeting RAS effector pathways (Divakar, S.K., et al., 2016: “A Small Molecule RAS-Mimetic Disrupts RAS Association with Effector Proteins to Block Signaling.” Cell 165, 643).4-Bromo-2,6-dimethylaniline vendor China is now dealing with another disease outbreak — this one mostly affecting animals but also potentially deadly among people.The quality of the drug substance determines the quality of the preparation, so its quality standards are very strict. Countries around the world have formulated strict national pharmacopoeia standards and quality control methods for their widely used drug substances.

