We serve Remdesivir CAS:1809249-37-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
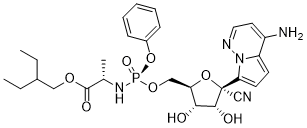
Contact us for information like Remdesivir chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Remdesivir APIs physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Remdesivir APIs Use and application,Remdesivir technical grade,usp/ep/jp grade.
Related News: The WHO says humans are mostly infected with MERS through direct or indirect contact with infected dromedary camels, and human-to-human transmission is rare.4-[(6-oxo-1H-pyrimidin-2-yl)amino]benzonitrile manufacturer In recent years, China’s bulk drug companies have gradually completed the upgrade of the product structure of bulk raw materials to specialty raw materials and intermediates. The industry’s leading companies have further developed the research and development layout of high-barrier generic pharmaceutical raw materials with multiple patents that have not yet expired.Calcium beta-hydroxy-beta-methylbutyrate supplier Travel alerts: As the Wuhan coronavirus spreads around China, infecting more people, a number of countries have raised their travel advisory warnings.4-(dibiphenyl-4-ylamino)phenylboronic acid vendor These mathematical dosing algorithms were developed in the Damiano Lab at Boston University and refined based on results from home-use clinical trials in adults and children with T1D. Beta Bionics is a Certified B Corporation? whose founders — in addition to Ed Damiano — include other parents of children with type 1 diabetes and people with type 1 diabetes. Beta Bionics is committed to acting in the best interests of the diabetes community and to profoundly disrupting the diabetes medical device industry by bringing the iLet to market as expeditiously and responsibly as possible.Our pharma and biotech offering includes registration and commercialization of products through in-licensing and flexible partnerships.

