We serve Sodium Sulfate CAS:7757-82-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
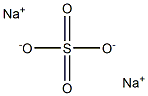
Product Name:Sodium Sulfate
Synonym: /
CAS NO.:7757-82-6
Chemical & Physical Properties
Density: 2.68 g/mL at 25 °C(lit.)
Boiling Point: 1700°C
Melting Point: 884 °C(lit.)
Molecular Formula: Na2O4S
Molecular Weight: 142.042
Exact Mass: 141.931274
PSA: 88.64000
Vapour Pressure: 3.35E-05mmHg at 25°C
Index of Refraction: 1.484
Storage condition: Store at RT.
Stability: Stable. Incompatible with strong acids, aluminium, magnesium, strong bases. Hygroscopic.
Water Solubility: 18.5 mg/L
Specification
Appearance: White powder
Assay: ≥98.0%
Water insoluble matter: ≤0.1%
Ca-Mg: ≤0.3%
Chloride: ≤0.7%
Fe: ≤0.01%
Water : ≤0.5%
Application
Mainly used as a filler for dyes and auxiliaries to adjust the concentration of dyes and auxiliaries so that the standard concentration can be achieved. It can also be used as a dye accelerator for direct dyes, sulfur dyes, and vat dyes when dyeing cotton, as a retarder for direct acid dyes when dyeing silk and wool animal fibers, and as a background color for printing silk fabrics. protective agent, etc.
Package and Storage
25kg/bag, store at room temperature away from light.
Contact us for information like Sodium Sulfate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Sodium Sulfate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Sodium Sulfate Use and application,Sodium Sulfate technical grade,usp/ep/jp grade.
Related News: Most foreign drugmakers offered Beijing the lowest prices globally in a recent round of negotiations in order to get some of their new products included in national insurance program, a move that will help them gain access to more patients in less-affluent cities, officials said on Thursday.Butanedioic acid, bis(3-oxo-4-pentenyl) ester manufacturer “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.1,3-Diaminobenzene Sulfate supplier “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.7,8-dimethoxy-1,2,3,5-tetrahydro-3-benzazepin-4-one vendor “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.“Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.

