We serve tert-Butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate CAS:571188-59-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
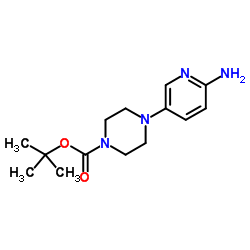
Contact us for information like 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate Use and application,tert-butyl 4-(6-aminopyridin-3-yl)piperazine-1-carboxylate technical grade,usp/ep/jp grade.
Related News: Over the years, large international pharmaceutical companies have gradually focused on the research and development and sales of patented drugs, and have gradually outsourced traditional products. Among them, most of the contractors are pharmaceutical companies in emerging countries.1-Chlorooctadecane manufacturer The Company’s first-of-kind approach involves engineering human iPSCs in a one-time genetic modification event and selecting a single engineered iPSC for maintenance as a clonal master iPSC line.tert-butyldimethylsilyl chloride supplier Health officials said it remained unclear where he had developed the disease.4-Aminophenol sulfate vendor High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies.In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.

