We serve tert-Butyl isothiocyanate CAS:590-42-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
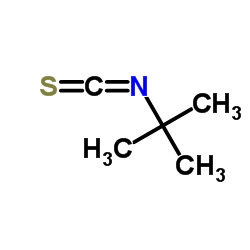
Contact us for information like tert-Butyl isothiocyanate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-isothiocyanato-2-methylpropane physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-isothiocyanato-2-methylpropane Use and application,2-isothiocyanato-2-methylpropane technical grade,usp/ep/jp grade.
Related News: To date, eight cases have been confirmed in the US: three people in California, two in Illinois and one individual each in Massachusetts, Washington State, and Arizona.1,3-Dichloro-5-fluorobenzene manufacturer The drug substance is an active product that has completed the synthetic route, and the intermediate is a product in one place in the synthetic route.L-Alanyl-L-Glutamine supplier As part of the agreement, ICIG will enter into a 5-year supply contract to provide Genzyme with materials needed for the production of eliglustat tartrate, an investigational treatment for Gaucher disease Type 1 that is currently in Phase III clinical trials.4-Bromophenylboronic acid vendor Over the years, large international pharmaceutical companies have gradually focused on the research and development and sales of patented drugs, and have gradually outsourced traditional products. Among them, most of the contractors are pharmaceutical companies in emerging countries.Only when the drug substance is processed into a pharmaceutical preparation can it become a drug for clinical application.

