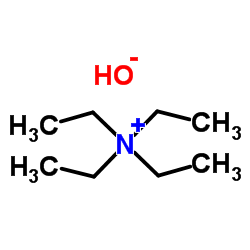
We serve Tetraethylammonium hydroxide CAS:77-98-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
Contact us for information like Secretin Acetate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,TEAH physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Teduglutide Use and application,Teduglutide technical grade,usp/ep/jp grade.
Related News: On 23 January, Chinese authorities confirmed this nCov can be transmitted between humans, with dozens of medical staff contracting the virus after treating infected patients.2-Chloro-5-fluorobenzaldehyde manufacturer On 23 January, Chinese authorities confirmed this nCov can be transmitted between humans, with dozens of medical staff contracting the virus after treating infected patients.2,4-Bis(trifluoromethyl)aniline supplier Large pharmaceutical companies are investing a disproportionately large amount of annual revenue in research and development (R&D), according to GlobalData’s Company Financials database.1-Bromo-8-chloronaphthalene vendor Large pharmaceutical companies are investing a disproportionately large amount of annual revenue in research and development (R&D), according to GlobalData’s Company Financials database.“Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.

