We serve trans,trans-2,4-Decadien-1-al CAS:25152-84-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
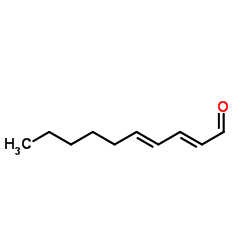
Contact us for information like trans,trans-2,4-Decadien-1-al chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(2E,4E)-Deca-2,4-dienal physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(2E,4E)-Deca-2,4-dienal Use and application,DDA technical grade,usp/ep/jp grade.
Related News: Fate Therapeutics, Inc. recently announced new in vivo preclinical data for FT819, its first off-the-shelf, iPSC-derived chimeric antigen receptor (CAR) T-cell product candidate, at the 61st American Society of Hematology (ASH) Meeting and Exposition in Orlando, FL.5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-amine manufacturer DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation.2-(4,6-Diphenyl-1,3,5-triazine-2-yl)-5-[(hexyl)oxy]phenol supplier DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation.3-Acetyl-5-chlorothiophene-2-sulfonamide vendor Both were in Siberia, and both are Chinese nationals who had recently traveled to China.Nevertheless, many other didn’t appear to need confirmation. Posts on Weibo, China’s Twitter-like platform, purportedly showed people lining up at night outside pharmacies across China to buy Shuanghuanglian.

