We serve β-Nicotinamide adenine dinucleotide CAS:606-68-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
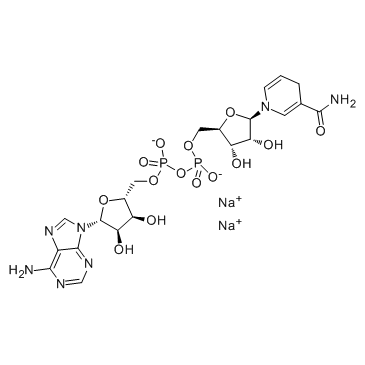
Contact us for information like Polyinosinic-polycytidylic acid sodium chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,beta-NADH disodium salt physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,β-Nicotinamide adenine dinucleotide Use and application,β-Nicotinamide adenine dinucleotide technical grade,usp/ep/jp grade.
Related News: Fate Therapeutics’ iPSC product platform is supported by an intellectual property portfolio of over 250 issued patents and 150 pending patent applications.5-fluorocytidine manufacturer Fate Therapeutics’ iPSC product platform is supported by an intellectual property portfolio of over 250 issued patents and 150 pending patent applications.4-aminobenzenesulfonic acid supplier Fate Therapeutics’ iPSC product platform is supported by an intellectual property portfolio of over 250 issued patents and 150 pending patent applications.2,3-Dichloro-5,6-dicyano-1,4-benzoquinone vendor The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.

